Product Responsibility: What To Know Before You Buy

Here / Boyloso / Anna Tryhub / Shutterstock.com
Did you wash your hands?
The Centers for Disease Control (CDC) and Prevention’s published guidance for hand sanitation is to wash with soap and warm water for a minimum of 20 seconds. But the lack of access to water, when needed, and today’s mobile lifestyles can thwart our efforts to maintain good hand hygiene, leaving us exposed to germs and other infectious agents. The convenience and portability of hand sanitizer is a suitable alternative that works well just about anywhere.
This article provides a brief history detailing the applicable regulations and labeling requirements, potential challenges for companies pivoting to production of hand sanitizer during the current pandemic and common reasons for hand sanitizer recalls, along with best practices.
![]()
Ethyl alcohol (also referred to as ethanol or, commonly, as alcohol) has been used as an antiseptic since the Middle Ages. Alcohol-based disinfectant hand rubs (the medical term for hand sanitizers) are also listed among the safest and most effective medicines used in modern health-care systems. In the United States, S. C. Johnson & Son, Inc. owned the patent for a skin moisturizing/conditioning antimicrobial alcoholic gel until 2009, when the patent expired.
Since the start of the pandemic earlier this year, the Food and Drug Administration (FDA) has recommended a 60-75-percent ethanol-based solution that largely follows the formula of the original patent.
Alcohol-free hand sanitizers are also commercially available. These products may contain povidone-iodine, benzalkonium chloride or triclosan as active ingredients. Alcohol-free sanitizers are, however, susceptible to contamination and have been subject to recalls for bacterial contamination. For this reason, the CDC continues to advocate alcohol-based formulas.
![]()
Hand sanitizer is regulated as an over-the-counter (OTC) drug by the U.S. Food and Drug Administration (FDA). The FDA regulates not only the contents of such products but also the conditions under which these products may be manufactured. (Note: The FDA defines drug as “(a) substance intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease.” Hand sanitizers aid in the prevention of disease.)
The Food and Drug Act of 1906 required manufacturers to accurately identify a product’s content and concentration as well as the volume or count of the product contained in the package. The FDA’s mandate expanded in 1938 to include cosmetics and medical devices. The new scope included standardization of the drug approval process (pre-market screening) and added a requirement that all drugs be labeled with safe dosage and usage information. The broadened mandate also laid the groundwork for modern FDA factory inspections and certifications.
Literally thousands of drug products have been removed from the U.S. market as a result of the enactment of these regulations because those drugs lacked evidence of safety and/or effectiveness.


All companies involved in any aspect of the manufacturing, packaging or labeling of an OTC drug product are required to register with the FDA (see box below for this link and others).
While a more rigorous regulatory process is in place for prescription drugs, the FDA maintains well-defined procedures for classifying OTC drugs as safe and effective. Manufacturers are required to detail the quantities of active ingredients contained in the recommended dosage—or, in the case of hand sanitizer, an application—providing animal and human safety studies (controlled, partially controlled and uncontrolled) relating to the individual active ingredients, combinations of the active ingredients and the finished product.
Even if a formula is deemed safe, potential for contamination or other harm exists in how the product is manufactured, packaged or labeled. The FDA requires all participants in the OTC supply chain to adhere to Current Good Manufacturing Practices (cGMP) (21 CFR Parts 1-99, 200-299, 300-499, 600-799 and 800-1299) validated through an onsite audit that includes a review of production records, traceability of manufactured product and a recall process.

Because of an increased demand for hand sanitizer during the coronavirus pandemic, the FDA has issued two Emergency Use Authorization (EUA) letters. The EUAs still effectively require new participants in the hand sanitizer supply chain to adhere to all existing regulations.
The first permits existing drug compounders to produce ethyl alcohol-based hand sanitizer for public distribution.
The second letter (FDA-2020-D-1106), relevant to the promotional products industry, allows new manufacturers, repackagers and distributors of alcohol-based hand sanitizers to temporarily register their manufacturing facilities and their hand-sanitizer products, as long as they adhere to both United States Pharmacopeia (USP) guidance for ingredients and formulas, and the WHO Guide to Local Production. Per the letter, the FDA is waiving validation enforcement actions. Violative, unsafe product remains subject to recall.
![]()
Labels inform the end user about the product, how it is intended to be used and the cautions to be exercised.
For hand sanitizer, labels not only perform this function for the consumer, but also for other members of the supply chain.
![]()
The FDA published the final OTC Drug Facts Label regulation in March 1999. The following information, in this order, must appear on all OTC product distributed into the market:
- The product’s active ingredients, including the amount in each dosage unit.
- The purpose of the product.
- The uses (indications) for the product.
- Specific warnings, including when the product should not be used under any circumstances, and when it is appropriate to consult with a doctor or pharmacist.
- This section also describes side effects that could occur and substances or activities to avoid.
- Dosage instructions: when, how and how often to take the product.
- The product's inactive ingredients, important information to help consumers avoid ingredients that may cause an allergic reaction.
The main drug facts title, all headings, subheadings and other information must be printed in a single, clear, easy-to-read type style with no more than 39 characters per inch. Titles and headings should be bolded italic with subheadings in bold only. Headings should be no smaller than eight-point type, while subheadings and all other information should be no smaller than six-point type.
The approved format of the label is as shown in Figure 1.


Ensuring safe usage of any drug product starts with understanding who the ultimate customer is, how the product is intended to be used and the properties of the drug’s ingredients.
The ethyl alcohol base of hand sanitizers, despite its efficacy as an antiseptic and disinfectant, poses a number of hazards for those who may come in contact with the product.
Ethyl alcohol is highly flammable and categorized as a Class 3 Dangerous Good (DG). A 65-70-percent (v/v) solution (the concentration common among most promotional hand sanitizers) has a flashpoint of 73° Fahrenheit or 23° Celsius.
In environments with high exposure to alcohol, alcohol can cause skin and eye irritation. Ingesting alcohol can cause drowsiness and dizziness and potentially damage internal organs. Alcohol also poses an inhalation risk.
With these known hazards, hand-sanitizer labels should include the following warnings:
- For external use only
- Do not use on open skin wounds
- Avoid contact with eyes
- May be harmful if swallowed
- Keep out of the reach of children
- For use by children only under adult supervision
- Do not use on children less than two months of age
- Flammable: keep away from flame and heat
Additionally, all hand sanitizer should be marked with a lot code or batch number and expiry date.

The Occupational Safety and Health Administration (OSHA) provides guidance to industry for managing risk associated with chemical hazards, such as those associated with ethyl alcohol. For manufacturing and storage facilities where hand sanitizers are either manufactured and the finished product or the ingredients for the product are stored, in addition to cGMPs, the facility should be climate-controlled and properly ventilated. No open flames or smoking should be permitted in areas where alcohol is stored or used in the production process.
Employees engaged in manufacturing operations in the areas where ingredients are mixed or pre-mixed solution is transferred to subcontainers should be provided with appropriate personal protective equipment (PPE) that may include gloves, safety glasses and respirators.
Labeling all bulk containers of ethyl alcohol with Safety Data Sheet labels ensures employees are aware of the contents and the necessary precautions to be taken. (See Figure 2)
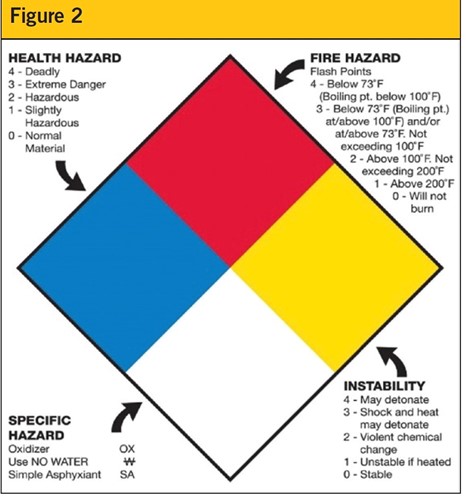

The Department of Transportation’s Pipeline and Hazardous Materials Safety Administration (PHMSA) regulates the transportation of hazardous substances known as Dangerous Goods, a global standard for hazardous materials established by the United Nations.
Due to fire hazards posed by ethyl alcohol, packages containing alcohol-based hand sanitizer are restricted from air freight and limited in how they may be packed for surface transport.
49 CFR § 173. 150(g) permits transportation of products containing no more than 70-percent ethyl alcohol by volume for liquids. The outer package, in addition to the company’s name, must be labeled with a Dangerous Goods label identifying the contents as sanitizer containing ethyl alcohol, a flammable liquid. Packaging must be leak-tight and securely closed, with interior contents protected from damage.
During the pandemic, PHMSA has issued the Temporary Policy for the Transportation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19). Find it here: www.phmsa.dot.gov/sites/phmsa.dot.gov/files/2020-04/PHMSA%20Hand%20Sanitizer%20Relief%20Notice.pdf.
Some flexibility in packaging configuration is outlined in the letter, however, key provisions for all packaging in which hand sanitizer is shipped to your company or to your customer include the following:
- Must be overpacked in crates, cages, carts, boxes or similar overpacks.
- Packages are secured in the transport vehicle in such a way as to prevent breakage, leakage and movement. Packages are packed with package closures in an upright orientation.
- The company name and the words ''Sanitizer - Contains Ethyl Alcohol'' or ''Sanitizer - Contains Isopropyl Alcohol'' is marked on the outside of the single package and the overpack.
- Air shipments remain strictly prohibited.

Hand sanitizer usage seems rather straightforward: Apply a palmful of alcohol-based hand sanitizer. Cover all surfaces of the hands. Rub hands until dry.
![]()
In 2017, the CDC published a paper titled “Reported Adverse Health Effects in Children from Ingestion of Alcohol-Based Hand Sanitizers – United States, 2011 – 2014.” The paper used data collected from the National Poison Data System and examined intentional and unintentional ingestion of hand sanitizer and the health risks to children 12 years of age and under.
There were 65,293 reported incidents of ingestion of alcohol-based hand sanitizer during the period reviewed. Approximately 90 percent of the incidents occurred among young children aged 0-5 years, with 97 percent of those cases involving oral ingestion.
Hand sanitizers frequently contain colorants, scents and other additives that make the gel attractive to children.
Researchers determined older children were more likely to intentionally ingest the sanitizer, suggesting this might be potential product abuse. Older children reported more symptoms and had worse health outcomes than younger children.
Common Symptoms
- Drowsiness
- Eye irritation
- Conjunctivitis
- Sore throat
- Cough
- Stomachache
- Nausea
- Vomiting
Rare Health Effects
- Coma
- Seizures
- Hypoglycemia
- Metabolic Acidosis
- Respiratory depression
Many manufacturers of hand sanitizers add a bitterant such as Bitrex to mitigate the unintentional or intentional risk of a consumer self-poisoning.
![]()
The active ingredient in the hand sanitizer manufacture and distribution exempted under the FDA’s EUA is ethyl alcohol, a flammable liquid. While alcohol is quick to evaporate, the user is directed to “rub hands until dry” and warned against exposure to a flame. Yet chemical burns remain one of the common injuries associated with hand sanitizers as reported in the National Electronic Injury Surveillance System (NEISS).
Ethyl alcohol, when ignited, produces a blue flame. A 2017 YouTube sensation involved dipping the user’s fingers in hand sanitizer then lighting the hand sanitizer-coated fingertips. The quick-burning fuel, supposedly, burns out before singeing the skin.
As with many YouTube stunts, a warning should have accompanied the videos. The resulting injuries can range from first-degree burns to deeper second-degree or even third-degree burns.

Hand hygiene is an important component of the U.S.’s response to the COVID-19 pandemic.
At this writing, the FDA has recalled more than 150 hand sanitizer products contaminated with or containing the chief ingredient methyl alcohol, also known as methanol. (Some recalled hand sanitizers’ contents have tested up to 81-percent (v/v) methanol.)
Methyl alcohol smells and appears similarly to ethyl alcohol. In contrast to ethyl alcohol, methyl alcohol is highly toxic and burns a white flame. Common usages of methyl alcohol are as a precursor chemical for the production of formaldehyde and other specialized chemicals.
Methyl alcohol should never be used in a hand sanitizer. The toxins attack the central nervous system, depressing breathing and heart rate. Symptoms include nausea, vomiting, headaches, permanent blindness and seizures among other harmful effects, up to and including coma and death.

The FDA recalls hand sanitizers for reasons beyond contamination. Recent hand sanitizer recalls include:
- Inadequate concentrations of ethyl alcohol
- The minimum concentration required for efficacy is 60 percent v/v
- Insufficient benzalkonium chloride (alcohol-free sanitizer)
- This antimicrobial is not approved for fighting COVID-19
- Hand sanitizers sold or offered for sale with false and misleading, unproven claims that the product can prevent the spread of viruses such as COVID-19, including claims the product can provide prolonged protection (e.g. for up to 24 hours)
- Products fraudulently marketed as “FDA-approved”
- Failure to comply with GMP requirements
- Verify your manufacturer’s registration in the FDA’s Inspection Classification Database Search at this link: www.accessdata.fda.gov/scripts/inspsearch/?utm_campaign=SBIA%3A%20Hand%20Sanitizers&utm_medium=email&utm_source=Eloqua

The CDC has identified ethyl alcohol-based hand sanitizers as the most effective hand sanitizer in the fight against coronavirus. When selecting a hand sanitizer for your client:
- Check the manufacturer’s / importer’s FDA registration
- Ask for the list of ingredients and the testing that validates content and product safety
- Check the label for active ingredients and warnings
- Check the packaging for batch number and expiry date
- Avoid colorants and fruity scents
- Avoid packaging that looks like liquor bottles
- Monitor hand sanitizer recalls
The FDA has also announced steps to address the gap in current testing protocols that allowed the flood of tainted hand sanitizer. An updated monograph for hand sanitizer was published for comment on July 31, 2020. Among the revisions is the addition of a methanol limit test to be added to the USP Alcohol and USP Dehydrated Alcohol monographs. Contact your laboratory service provider for more information.
The issues that have emerged around hand sanitizer during the current health-care crisis demand manufacturers and importers be especially diligent in adhering to compliance processes that protect the consumer.
Companies that are unfamiliar with FDA regulations for drug products should consider the regulatory requirements and the scope of the EUA and should then determine if compliance, under these specified conditions, is achievable.
D.E. Fenton was most recently executive director at Quality Certification Alliance. After 12 years, the organization concluded its mission and wrapped up operations as of August 1.
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
Resources
To register products with the FDA: www.accessdata.fda.gov/scripts/cder/training/OTC/topic4/topic4/da_01_04_0040.htm
Learn more about Current Good Manufacturing Practices (cGMP): www.fda.gov/drugs/pharmaceutical-quality-resources/facts-about-current-good-manufacturing-practices-cgmps
Emergency Use Authorization letter (FDA-2020-D-1106): www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-temporary-policy-preparation-certain-alcohol-based-hand-sanitizer-products-during
Guidance for local production: www.who.int/gpsc/5may/Guide_to_Local_Production.pdf?ua=1
www.fda.gov/drugs/coronavirus-covid-19-drugs/hand-sanitizers-covid-19
Drug labeling: www.fda.gov/drugs/drug-information-consumers/otc-drug-facts-label
https://www.osha.gov/dsg/hazcom/ghsguideoct05.pdf
USP Alcohol and USP Dehydrated Alcohol monographs: www.uspnf.com/notices/methanol-testing-nitr-20200731?utm_campaign=SBIA%3A%20Hand%20Sanitizers&utm_medium=email&utm_source=Eloqua
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
D.E. Fenton was most recently executive director at Quality Certification Alliance. After 12 years, the organization concluded its mission and wrapped up operations as of August 1.

