Product Responsibility: Facing The Facts About Face Masks

Tiko_photographer / Shutterstock.com
Face masks that help mitigate the transmission of the coronavirus are a common sight in the current coronavirus pandemic. Masks are one of many measures referred to as personal protective equipment (PPE). However, not all masks are considered PPE.
This article provides a brief history to help suppliers and distributors understand the applicable regulations and associated voluntary standards, the various types of face masks and facial coverings available and testing and requirements for PPE.
Regulatory Background
Most social accountability standards assess the proper deployment and use of personal protective equipment. PPE protects individuals in a workplace setting from a broad range of injuries and illnesses that may result from chemical, radiological, physical, electrical, mechanical or other such hazards, including biological hazards. PPE is worn to reduce employee exposure to hazards when engineering and procedural controls in the workplace are not feasible, or do not provide sufficient protection against a known hazard. Respirators, used to protect the wearer’s airways, have been regulated in the United States since 1919.
Currently, the Occupational Safety and Health Administration (OSHA), an agency within the Department of Labor, oversees these requirements. OSHA publishes guidelines for conducting a hazard assessment of the workplace, determining appropriate PPE and providing training on correct usage. Specific requirements for PPE are published in 29 CFR 1910. OSHA relies on standards established by the American National Standards Institute (ANSI) to define compliance requirements for many categories of PPE, including respirators and masks used in a workplace setting.
For certain masks and respirators, the Respiratory Protection Standard (29 CFR 1910.134), which is a standard that was enacted in 1988, regulates these types of PPE. Assigned Protection Factors (APF)—the workplace level of respiratory protection a device is expected to provide—were subsequently enacted in 2006. Following the Ebola crisis in 2015, ANSI Z88, which is the standard for respirators, was updated to reflect the needs of health-care workers. ANSI Z88 established the best practice minimum filtration as that provided by N95 respirator approved by the National Institute of Occupational Safety and Health (NIOSH). Government-published guidelines for masks and mask usage in health-care settings are found in the Hospital Respiratory Protection Program Toolkit (2015) issued jointly by the Department of Labor, OSHA, the Department for Health and Human Services, the Centers for Disease Control (CDC) and National Institute of Occupational Safety and Health (NIOSH). Access the publication at www.osha.gov/Publications/OSHA3767.pdf.
With the continuing health emergency, the United States has experienced a shortage of critical PPE. Through the issuance of Emergency Use Authorization (EUA) declarations, current Good Manufacturing Practices (GMP) requirements are suspended until the end of the current health-care crisis, or until otherwise communicated by the FDA. For more detail, read the National Law Review article here. This suspension includes the quality system requirements under 21 CFR Part 820 regarding design, manufacture, packaging, labeling, storage and distribution. Manufacturers are still required to ensure compliance with all manufacturing, product and marketing requirements, despite the procedures for submissions to the FDA being suspended at this time. Notably, NIOSH standards and requirements continue to be enforced. Guidance from the FDA has continued to evolve related to face masks and respirators.
Which Mask Is Right For Your Customer?
Ensuring the safety of any product for its intended use starts with understanding who the ultimate customer is and how the product is likely to be used.
The nature of the current crisis is that it is health-related and affecting society broadly. Normally, when considering which PPE to use, risk is limited to a workplace and perhaps a set of operations. Health-care providers require PPE, enabling them to more safely perform their job responsibilities, particularly in an infectious environment. Essential workers are also recommended to use PPE in their work. Due to the contagious nature of COVID-19, even people practicing social distancing are recommended to use PPE or other face coverings to limit the potential spread of the virus.
The NIOSH Hospital Respiratory Protection Program Toolkit provides these key definitions for face coverings intended to protect against the spreading of viruses:
Respirator: A device worn over the nose and mouth to protect the wearer from hazardous materials in the breathing zone. Respirators must be certified by NIOSH for the purpose for which they are used.
Surgical respirator: A filtering facepiece respirator with fluid-resistant face mask material on the outside to protect the wearer from splashes. Also known as a surgical N95 respirator, it meets a filtration efficiency level defined in 42 CFR 84.181.
N95 respirator: A general-use term for a half mask air-purifying respirator with NIOSH-approved N95 particulate filters or filter material that meets the filtration efficiency level defined in 42 CFR 84.181.
N95 filter: A type of NIOSH-approved filter or filter material, which captures at least 95 percent of airborne particles and is not resistant to oil.
Face mask: A loose-fitting, disposable device that creates a physical barrier between the mouth and nose of the wearer and potential contaminants in the immediate environment. Face masks may be labeled as surgical, laser, isolation, dental or medical-procedure masks and are cleared by the FDA for marketing. Face-mask filtration effectiveness is certified by NIOSH but varies widely among models. Face masks may come with or without a face shield. Face masks are not considered respiratory protection.
Surgical mask: A fluid-resistant, disposable and loose-fitting device that creates a physical barrier between the mouth and nose of the wearer and the immediate environment. For use in surgical settings, surgical masks do not provide the wearer with full protection from inhalation of airborne pathogens, such as viruses. The mask also meets Class I or Class II flammability tests.
Non-surgical mask: Used to protect patients and staff from the transfer of respiratory secretions, fluids or other debris. Procedure masks are used for general “respiratory etiquette” to prevent clinicians, patients and visitors from spreading germs by talking, coughing or sneezing. They are not intended for surgical use and are not considered PPE.
Facepiece: The part of a respirator that covers the nose and mouth of the wearer. Respirators may have half facepieces covering just the nose and mouth, or they may have full facepieces covering the nose, mouth and eyes. They are designed to form a seal with the face.
Loose-fitting facepiece: This is the portion of a respirator that forms a partial seal with the face but leaves the back of the neck exposed. It is designed to form a partial seal with the face through which clean air is distributed to the breathing zone.
Cloth face covering: This item is worn to fully cover the mouth and nose. Face coverings may prevent the wearer from spreading respiratory droplets when talking, sneezing or coughing. They are not considered PPE.
Hazard Assessment And Intended Use
The role of a hazard assessment is to identify potential risks of harm, the likelihood of exposure to the harm and potential mechanisms for mitigating the risk associated with the harm. Employing face masks and respirators that are designed to limit exposure to the specific hazard in the workplace improves worker safety and protection. Amongst the general public, face coverings may prevent the person wearing the covering from spreading respiratory droplets when talking, sneezing or coughing.
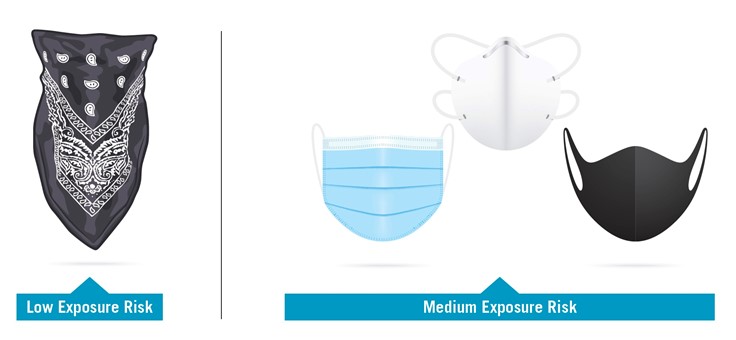
OSHA has developed a classification of exposure for airborne pathogens that corresponds with specific requirements for mask types for use in mitigating infection:
Low Exposure Risk: Jobs that do not require contact with people known to be, or suspected of being, infected. Workers have minimal occupational contact with the public and other coworkers.
Medium Exposure Risk: Jobs that require frequent or close contact with people who may be infected, but who are not known to have or suspected of having COVID-19.
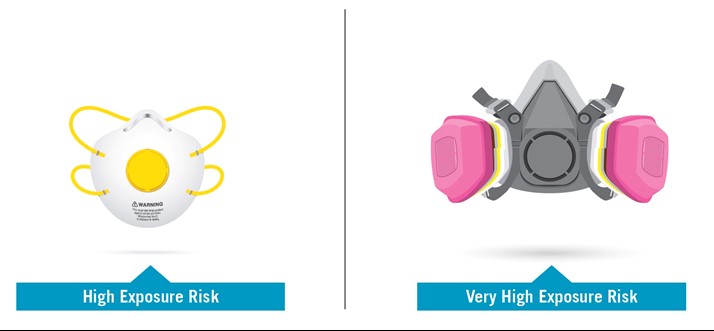
High Exposure Risk: Jobs with a high potential for exposure to known or suspected sources of SARS-CoV-2. (SARS-CoV-2 is the virus that causes COVID-19.)
Very High Exposure Risk: Jobs with a very high potential for exposure to known or suspected sources of SARS-CoV-2 during specific medical, postmortem or laboratory procedures.
Job duties affect exposure risk. As workers’ job duties change or they perform different duties during the day, they may move from one exposure level to another.
Meeting Compliance Standards As Companies Pivot to Manufacturing And Importing Face Masks
The standards that apply depend on the aspect of manufacturing, product design and the market in which the product is to be distributed.
For Masks Intended For Use In A Medical Setting
All masks and respirators intended for use in a medical setting are subject to FDA regulations.
Face masks are regulated as medical devices, under 21 C.F.R. Section 878.4040, when intended for medical purposes (non-surgical masks). Surgical masks are, likewise, regulated under 21 CFR 878.4040, but as class II devices requiring premarket notification.
Contingent upon whether the masks are intended for use by a health-care professional, a patient or a visitor, the masks may also be subject to OSHA regulation.
- For microbial claims, joint review by FDA and Environmental Protection Agency (EPA) is required.
- In environments where the risk of infection in the workplace to an employee is deemed high, masks are required to demonstrate compliance to NIOSH filtration standards. The facility is also required to implement a comprehensive PPE control program covering risk assessment, deployment, training, disinfection and disposal.
- Any marketing claims fall to the co-jurisdiction of the FDA and Federal Trade Commission (FTC).
For manufacturers and importers of masks intended to be distributed within this category:
- Register the production site. A Good Manufacturing Practices (cGMP) audit is required for completing the registration process with the FDA.
- Register the product. The mask, specifications, testing and packaging are submitted for review to the FDA (masks registered as PPE compliant to ANSI Z88 standards must also be submitted to NIOSH for certification). Standards include non-irritating body contact fabrication, compliance with Class I or Class II flammability per 16 CFR 1610 (unless labeled against use in environments with potential exposure to high-intensity heat {e.g. laser} or flammable gas {e.g. oxygen}) and fluid resistance barriers, all to be validated through testing.
- Manufacture lot coding and expiration dates are required.
- For single-use, disposable masks, exterior packaging and inner packaging of units must contain a printed description that includes the product name and a description of the product, the manufacturer name and location, the production lot code and an expiration date.
- For single-use N95 masks and respirators, a printed description on the mask that includes the product name and model number, the manufacturer’s name and location, the production code and expiration date is required. Additionally, NIOSH-registered masks require mask exterior markings including “NIOSH” in block letters, the Testing and Certificate (TC) approval number and a warning describing the cautions and limitations of usage.(NOTE: Any decoration on or modification to a NIOSH-approved model invalidates the TC approval.)
- Manufacture lot coding and expiration dates are required.
- Retain all relevant development, validation and production records for masks produced under the EUA until otherwise notified by the FDA. The FDA may, at any time, request inspection of all applicable records.
- For importers:
- Advise manufacturers of the above guidelines.
- Ensure all masks are correctly classified for importation.
- For non-medical masks: Clearly indicate product is not intended to be used in a health-care setting in the commercial documents and on the cartons.
- Avoid efficacy claims for disease prevention.
- Avoid antimicrobial / antiviral agents claims.
- For importers:
- Maintain an effective recall process which could be activated to withdraw product from the market that may pose a risk to the end user.
- Avoid marketing materials that may represent or suggest that the mask is safe or effective for the prevention of COVID-19.
- Keep in mind the EUA will be effective only until the declaration that circumstances exist justifying the authorization of the emergency use of personal respiratory protective devices during the COVID-19 outbreak is terminated under Section 564(b)(2) of the Act or the EUA is revoked under Section 564(g) of the Act.
For Face Masks Intended For General Use Manufactured Under EUAs
The FDA-published EUAs from March and April 2020 clearly distinguish general use “face masks” from non-surgical and surgical masks. General use products may not imply “antimicrobial or antiviral protection” or use marketing claims such as “for use such as infection prevention or reduction” or “for particulate filtration.”
 The FDA defines general-use face masks, whether they include or do not include a face shield, as covering the user’s nose and mouth. These face masks may or may not meet fluid barrier or filtration efficiency levels. General-use face masks also include cloth face coverings. Lastly, general-use face masks may be used for single or multiple uses and, if for multiple uses, they may be laundered or cleaned.
The FDA defines general-use face masks, whether they include or do not include a face shield, as covering the user’s nose and mouth. These face masks may or may not meet fluid barrier or filtration efficiency levels. General-use face masks also include cloth face coverings. Lastly, general-use face masks may be used for single or multiple uses and, if for multiple uses, they may be laundered or cleaned.
Under the current EUAs, the FDA has authorized manufacturers and distributors to “make face masks available with labeling that include(d) a description of the product as a face mask, including a list of the body-contacting materials (which does not include any drugs or biologics).” The EUA also advises authorized manufacturers and distributors of these products against labeling the product:
- as a surgical mask, to provide liquid barrier protection
- or use in any surgical setting or where significant exposure to liquid, bodily or other hazardous fluids, may be expected
- for use in a clinical setting where the infection risk level through inhalation exposure is high
- for use in the presence of a high intensity heat source or flammable gas
- for antimicrobial or antiviral protection or related uses or uses for infection prevention or reduction or related uses, or
- to include particulate filtration claims.
Find more information in the Face Mask Emergency Use Authorization (Letter), issued by the U.S. Food and Drug Administration on April 24, 2020, at www.fda.gov/media/136449 download.
Instructions on the cleaning and care, if applicable, must be provided to end users in hard copy or via an alternative format with instructions on how to access.
Similar to the manufacturing requirements for medical masks, the FDA requires manufacturers to maintain production records, traceability and a recall process.
The EUA provides guidelines for manufacturers and distributors, including manufacturers and distributors of promotional products, related to printed inserts and packaging. No claims should be made nor statements that suggest the product “is safe or effective for the prevention or treatment of patients during the COVID-19 pandemic.”
Companies unfamiliar with FDA regulations for devices should consider the regulatory requirements and the scope of the EUA and should then determine if compliance, under these specified conditions, is achievable.
The mask images in this article are for illustration purposes only.
–––––––––––––––––––––––––––––––––––––––––––––––––––––––––––
D.E. Fenton was most recently executive director at Quality Certification Alliance. After 12 years, the organization concluded its mission and wrapped up operations as of August 1.

